The Importance of Medical Device Packaging

Packaging is an essential component of medical device manufacturing, allowing manufacturers to deliver products to hospitals and medical facilities safely. Here’s a guide to the industry requirements and importance of this process.
Requirements for Medical Device Packaging
Medical device manufacturing companies must comply with industry regulations when designing their packaging solutions to keep products sanitized. Specifically, products must remain completely sealed. Packages are tested to ensure there are no holes or leaks, even those microscopic in size since dirt and germs can sneak in through tiny openings.
Additionally, the FDA requires labels. For example, some must include instructions on outer packaging with simple product labels on each individual sealed product. These labels must also specify that a product is sealed and sterile.
Why Medical Device Packaging Is Essential
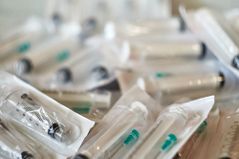 Medical devices often come in close contact with patients. Certain items like syringes may even come into contact with a person’s bloodstream, so contamination can affect a person’s health. Both accidental contact with dirt and germs and intentional tampering are concerns.
Medical devices often come in close contact with patients. Certain items like syringes may even come into contact with a person’s bloodstream, so contamination can affect a person’s health. Both accidental contact with dirt and germs and intentional tampering are concerns.
Additionally, medical device packaging is often responsible for displaying instructions for use, like sterilization procedures, potential safety concerns, or even ingredients. For example, if a product includes or has come into contact with latex, the packaging should warn against using the device on those with latex allergies.
If you’re looking for help with medical device manufacturing or packaging, Pacific Integrated Manufacturing in Bonita, CA, is here to help. The company offers plastic injection molding, assembly kitting, and assembly packaging. With more than 15 years of experience and a 5,000-square foot facility, the team is well equipped to handle projects quickly and effectively. Visit the website to see a full list of services. To request a consultation for medical device manufacturing or another service, call (619) 921-3464.
About the Business
Have a question? Ask the experts!
Send your question

