Deep Brain Stimulation | Guest Column by Kelly Kearney
With one of our CAP Board Members recently getting DBS, I wanted to find out for myself what this is all about, and how it can potentially help PWP’s. I was also interested in learning about DBS, as the MIT Research Initiative includes an MIT- specific brain device that can potentially have multiple uses (brain stimulation, brain fluid sampling, and drug delivery, as I understand it); that device is still in development and, of course, requires funding of the Initiative – we’re working with MIT on that, currently.
 I would like to thank Solomon Yi of Medtronic and Michael Barrs of Boston Scientific for being so generous with their time as they helped me to learn more about this important technology, and how each of their devices differ. (Abbott St. Jude is also in this space but I was unable to reach them before press time.) Bottom line: prospective patients need to do their homework and learn about each manufacturer’s products with an in-depth evaluation of the technology, the features and benefits, and the process from start to finish. We take no position on strengths/weaknesses, or recommendations.
I would like to thank Solomon Yi of Medtronic and Michael Barrs of Boston Scientific for being so generous with their time as they helped me to learn more about this important technology, and how each of their devices differ. (Abbott St. Jude is also in this space but I was unable to reach them before press time.) Bottom line: prospective patients need to do their homework and learn about each manufacturer’s products with an in-depth evaluation of the technology, the features and benefits, and the process from start to finish. We take no position on strengths/weaknesses, or recommendations.
Both Michael and Solomon expressed the same genuine level of interest in the wellbeing of Parkinson’s patients, and I want to make sure everyone knows that improvement in patient’s day to day was at the top of their lists. They are to be commended for that – both putting aside product differences to lobby for taking action, regardless of which solution is chosen.
With that in mind, and to remain objective and neutral, I decided to use the summary from MJFF that is just that: an even, objective overview that does not give advantage to any one manufacturer, but helps patients evaluate and become better informed about the technologies. So, with a shout out to MJFF for their content, here it is – direct from MJFF:
“The U.S. Food and Drug Administration (FDA) has approved deep brain stimulation (DBS) devices from three different manufacturers for Parkinson’s. While each device is unique, all DBS systems have the same basic components and work in a similar fashion. Each includes thin wires, or leads, implanted in the brain, and a neurostimulator and battery, implanted in the chest (or abdomen). Doctors program the neurostimulator to deliver small electrical pulses through the leads into areas of the brain that control movement in order to lessen Parkinson’s disease (PD) symptoms. The differences in devices are not drastic, but they represent innovation and widen treatment options.
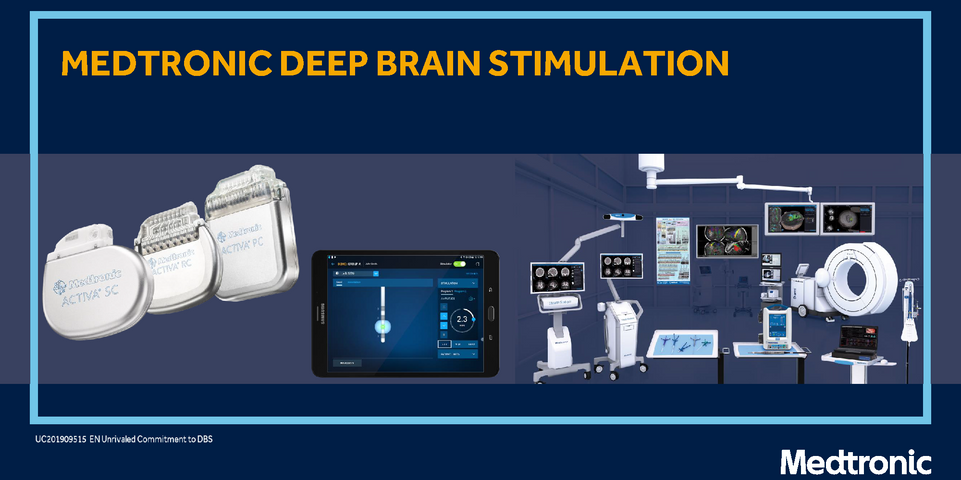
Medtronic Activa
Medtronic’s DBS was the first to be FDA-approved for PD, in 1997. Over the past two decades, Medtronic has added newer systems with rechargeable and non-rechargeable batteries. Rechargeable batteries may last up to 15 years, but require regular recharges. Non-rechargeable devices last, on average, about three to five years, depending on an individual’s settings. A person with Medtronic DBS can undergo most MRI scans safely when certain conditions are met.
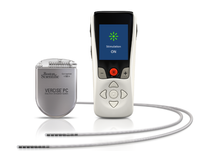
Boston Scientific Vercise
Boston Scientific’s Vercise became available for PD in Europe in 2012, and it gained FDA approval in 2017. Vercise’s brain leads contain more points (eight vs. four in other systems) through which doctors can precisely deliver and control electrical stimulation. Vercise uses a rechargeable battery, which may last up to 25 years. Similar to other DBS systems, Vercise is safe for most MRI scanning as long as several conditions are met.
Abbott St. Jude Medical Infinity
Abbott’s St. Jude Medical Infinity DBS was FDA-approved for Parkinson’s in 2016. The Infinity brain leads allow “directional stimulation,” which is a potentially increased ability to steer electrical stimulation toward symptoms and away from side effects. Abbott DBS operates with Apple iOS software and controllers (an iPad mini) for a possibly more familiar interface and easier programming experience. People with Abbott DBS may have most MRI scans safely when specific procedures are followed.
I highly recommend doing your homework and looking at all three; asking questions of movement disorder specialists, surgeons, and of course, other patients; and attending support group meetings, seminars, and other events where you can ask questions of the manufacturers, as well. There are lots of DBS seminars and support groups out there, please inquire if you aren’t sure of where to look and we can help.
If you have further questions, please let me know. kelly@parkinsonsct.org
About the Business
Have a question? Ask the experts!
Send your question

